Felycin®-CA1
(sirolimus delayed-release tablets)
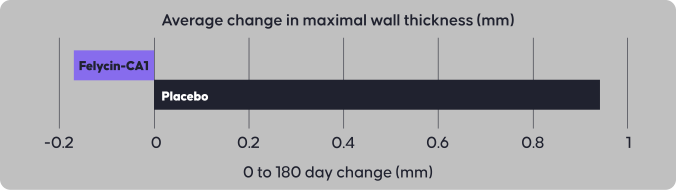
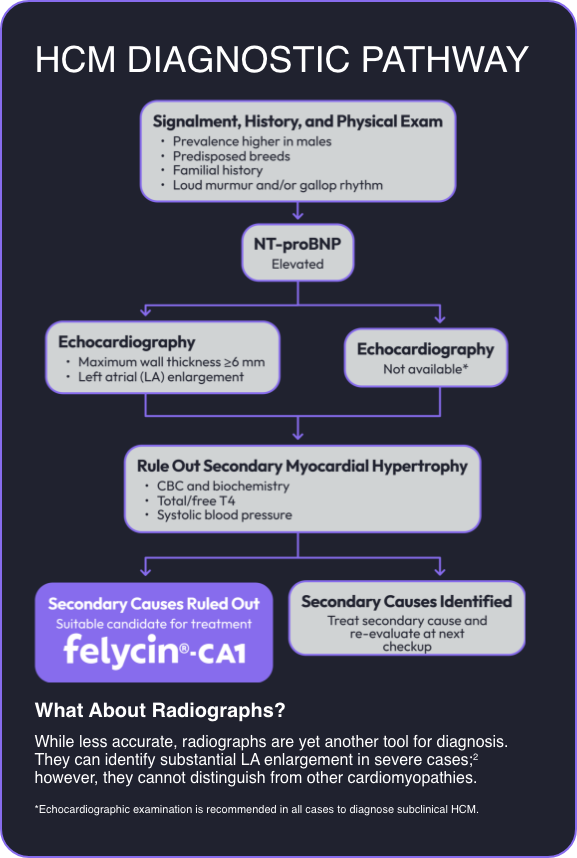
Felycin®-CA1 is the first FDA conditionally approved once-weekly drug for the management of ventricular hypertrophy in cats with subclinical hypertrophic cardiomyopathy (HCM).
Indication
Felycin-CA1 is indicated for management of ventricular hypertrophy in cats with subclinical HCM.
Available As
Tablet
For use with
Feline
Strengths
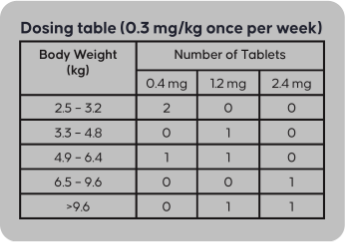
0.4 mg, 1.2 mg, or 2.4 mg tablet
Benefits
For the first time we can manage the disease
Felycin-CA1 is sirolimus delayed-release tablets for the management of ventricular hypertrophy in cats with subclinical HCM.
Conditionally approved by the FDA
It’s the first and only product conditionally approved by the FDA for use in cats with subclinical HCM.
Easy once-weekly administration for clients
The medication can be administered orally once weekly with or without the use of a pet piller.
Three strengths for dosing accuracy
Felycin-CA1 is a pill that comes in three strengths (0.4 mg, 1.2 mg, 2.4 mg).
About Felycin-CA1
ABOUT HCM
HCM is the most prevalent heart disorder in cats, and 1 in 7 cats has HCM. 1,2
References
1 Fuentes VL, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. JVIM 2020;34:1062-1077.
2 Kittleson MD, Cote E. The feline cardiomyopathies: Hypertrophic cardiomyopathy. JFMS 2021;23:1028-1051.